pipeline-resources
How to interpret the shotgun report
This document describes how to understand the bioinformatics report generated by Aladdin shotgun pipeline. Most of the plots are taken from the sample report. The sample report was generated using a small number of samples from this paper. The plots in your report might look a little different.
Table of contents
- Table of contents
- Report overview
- General statistics table
- Taxonomy Assignment
- Diversity analysis
- Differential abundance analyses
- Antimicrobial Resistance Analysis
- Sample processing
- Pipeline information
Report overview
The bioinformatics report is generated using MultiQC. There are general instructions on how to use a MultiQC report on MultiQC website. The report itself also includes a link to a instructional video at the top of the report. In general, the report has a navigation bar to the left, which allows you to quickly navigate to one of many sections in the report. On the right side, there is a toolbox that allows to customize the appearance of your report and export figures and/or data. Most sections of the report are interactive. The plots will show you the sample name and values when you mouse over them.
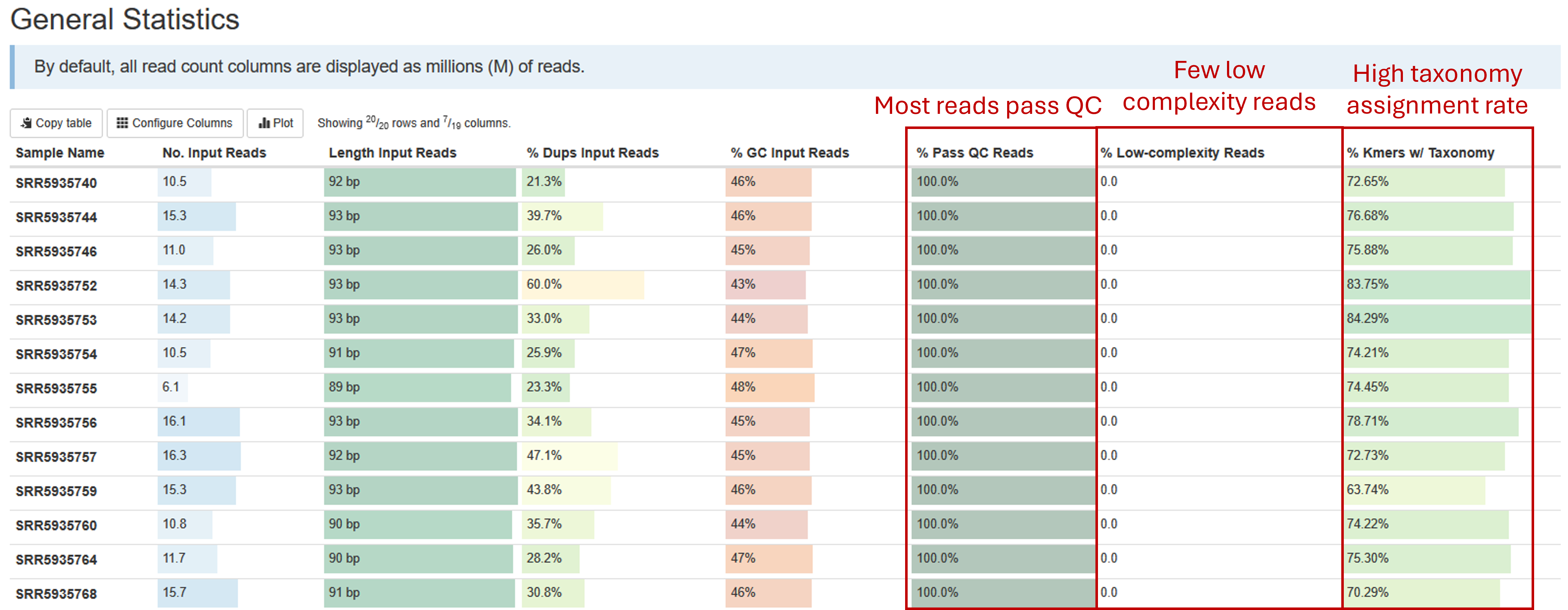
General statistics table
The general statistics table gives an overview of some important stats of your samples. They include some statistics of the input files, such as how many reads are in each sample, the lengths, duplication rate, and GC contents of the reads. They also tell you how many of your reads passed read quality QC (% Pass QC Reads), the percentage of reads that are of low complexity and therefore filtered (% Low-complexity Reads), and most importantly, percentages of reads/kmers that have assigned taxonomy (% Kmers w/ Taxonomy). The default sourmash method assigns taxonomy to kmers, instead of reads. But since kmers are generated from reads, the percentages of kmers with taxonomy can be approximated to percentages of reads with assigned with taxonomy.
A good shotgun sequencing library should have most reads pass QC, with few low-complexity reads. Most well-studied bacterial community, such as human gut microbiome samples, should have most reads with taxonomy assigned. A low taxonomy assignment rate could suggest possible host DNA contamination, which you will be able to investigate in a later chart. Some poorly-understood microbial communities could have unknown microbes without representations in the database, which could lead to low taxonomy assignment rate.
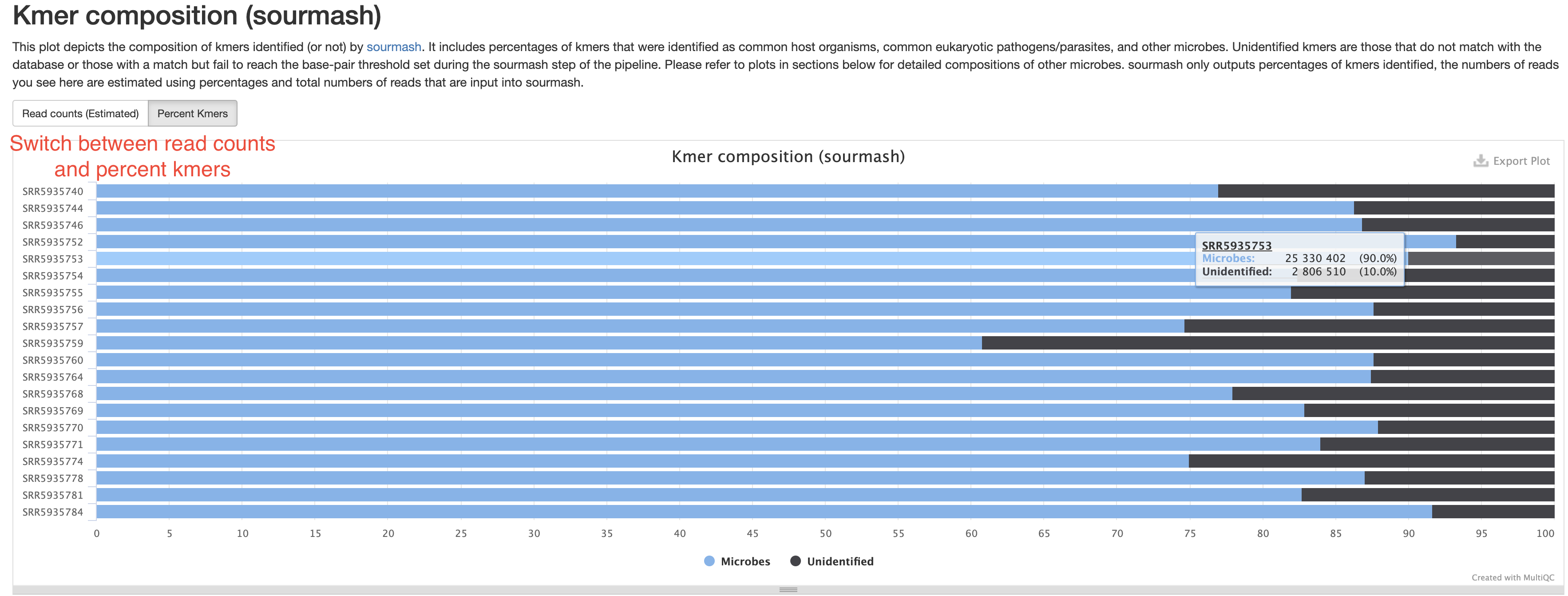
Taxonomy Assignment
Kmer composition (sourmash)
This section summarizes the percentages of kmers, or by approximation, the numbers of reads assigned with taxonomy. Those without any taxonomy assignment are labeled as “Unidentified”, those coming from host sources or common eukaryotic pathogen will be labeled by their species name, for example, homo sapiens. All reads assigned to microbes will be combined and labeled “Microbes”. This is supposed to give you a quick answers to a few questions:
- Were there any host sequences in my sample and how much? This helps assess the sampling quality and how much of the sequencing data were useful.
- Were there any eukaryotic pathogens detected?
- How much of the reads have no assigned taxonomy. There could be several reasons for this. For example, they could be from organisms without representative genomes in the database. They could also be from species with very low abundance. This is because
sourmashhas a reporting threshold (default 50kb). Only species with enough reads to reach this threshold will have a reported kmer percentage. Low abundance species have fewer reads, which may result in their reads not reaching the threshold.
In the sample report, there are no host or pathogen sequences. If they are present in your samples, they will also show up in this plot. The samples here are all gut microbiome samples, which contain mostly well known organisms, therefore most of the kmers have assigned taxonomy.
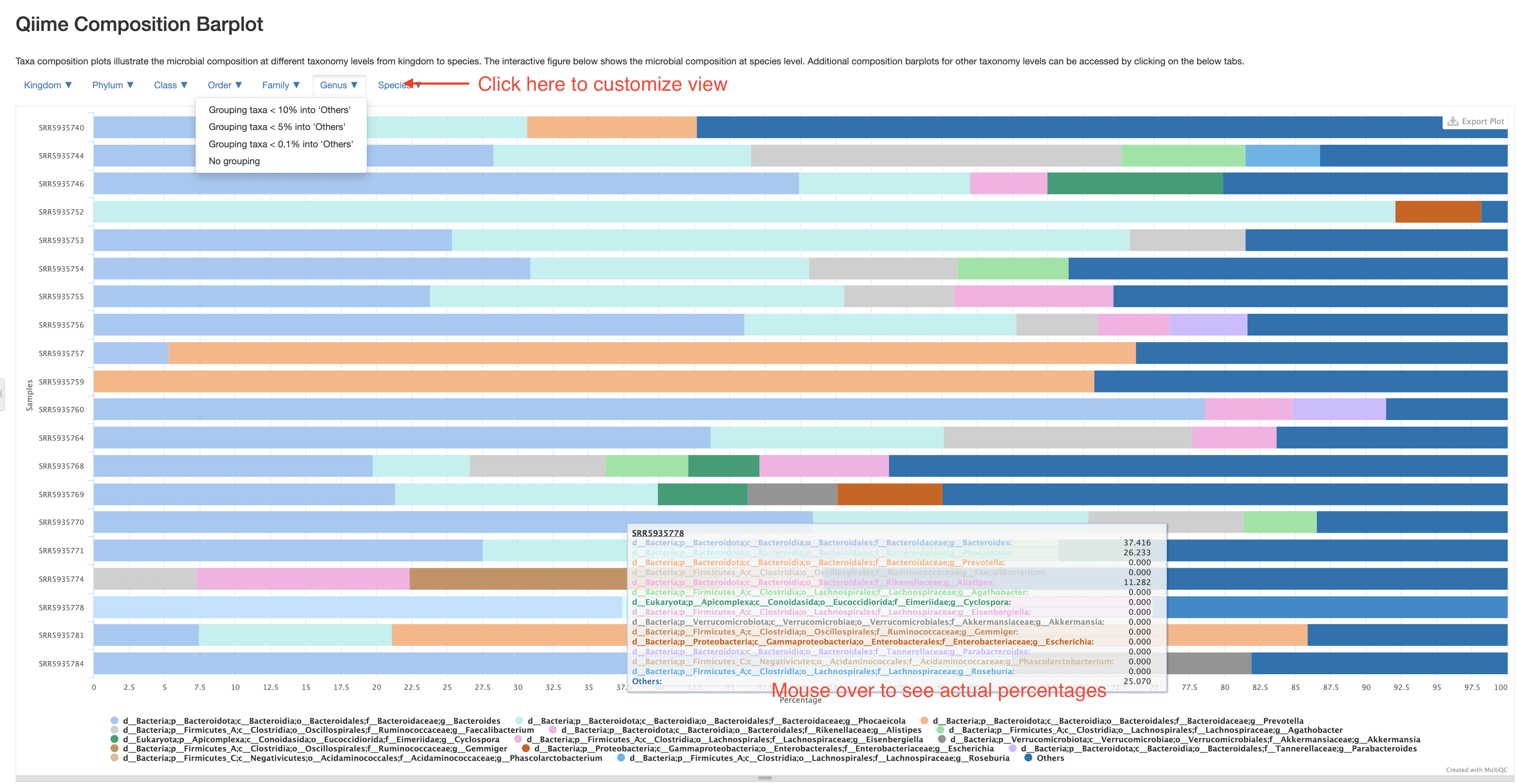
Qiime composition barplot
This section allows you to investigate the composition of every sample on different taxonomy levels. Please note, these composition barplot only accounts for reads/kmers assigned to microbes, those from hosts or without assigned taxonomy have already been excluded. Therefore, the percentages presented here are “percentage among known microbial reads”, not “percentage of all reads”. The plot starts with a species-level presentation that include all species. It could be very busy and not very informative. That is why we’ve made the plot interactive where you could switch to different taxonomy levels, and group less abundant taxa into an “Others” category. Please click the buttons at the top left corner of the plot to customize your view. For example, below is a presentation at Genus level with genera below 5% grouped into “Others”.
Krona Plot
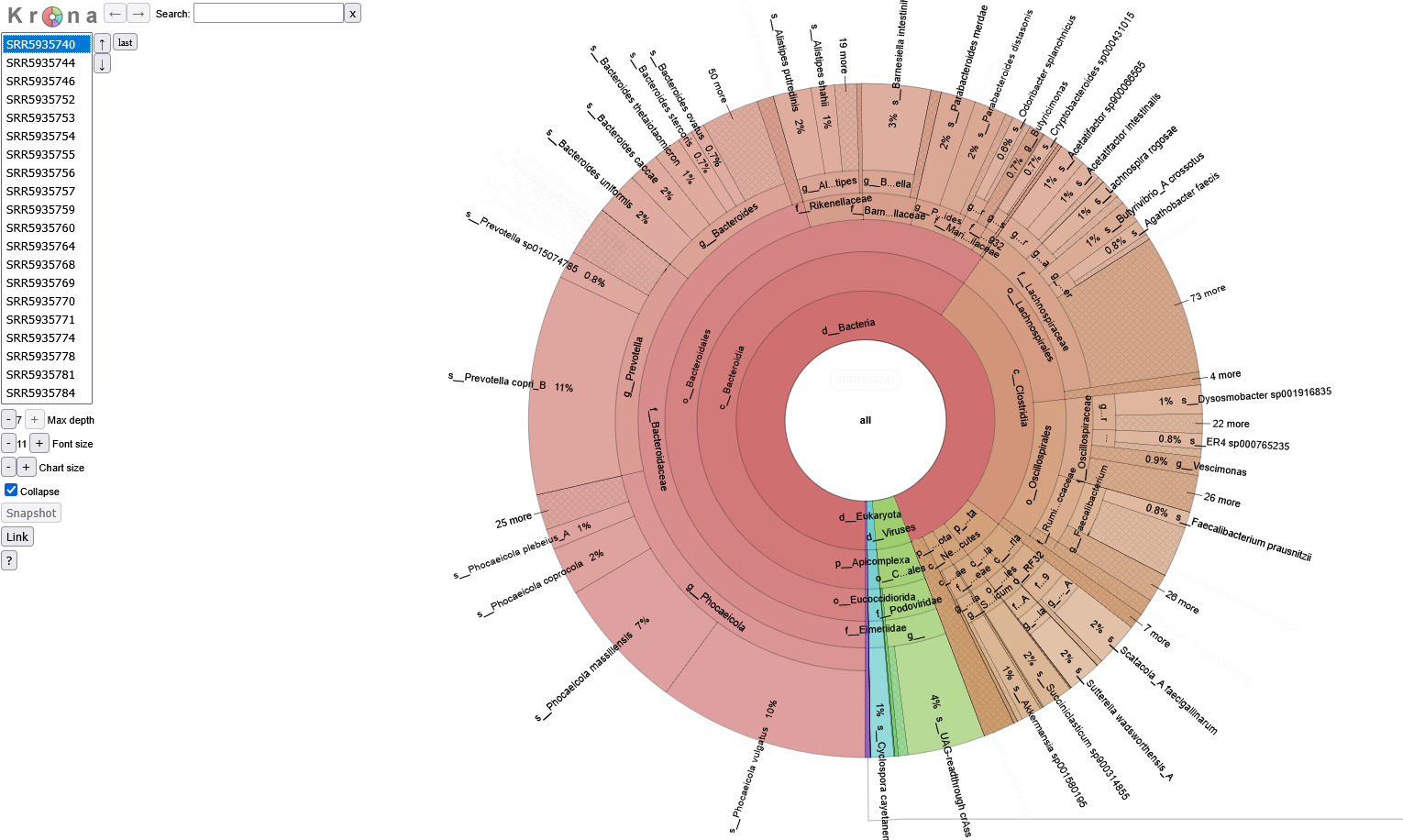
In some cases, numerous taxa identified in one sample can contribute to overcrowding in the composition barplots. If a more detailed format is desired, the pipeline also produces downloadable krona reports for each sample. Krona reports display the taxa composition of each sample as a pie chart. Use the table on the left hand side of the screen to select a sample. Mouse over a desired taxa and click on the highlighted section to expand it.
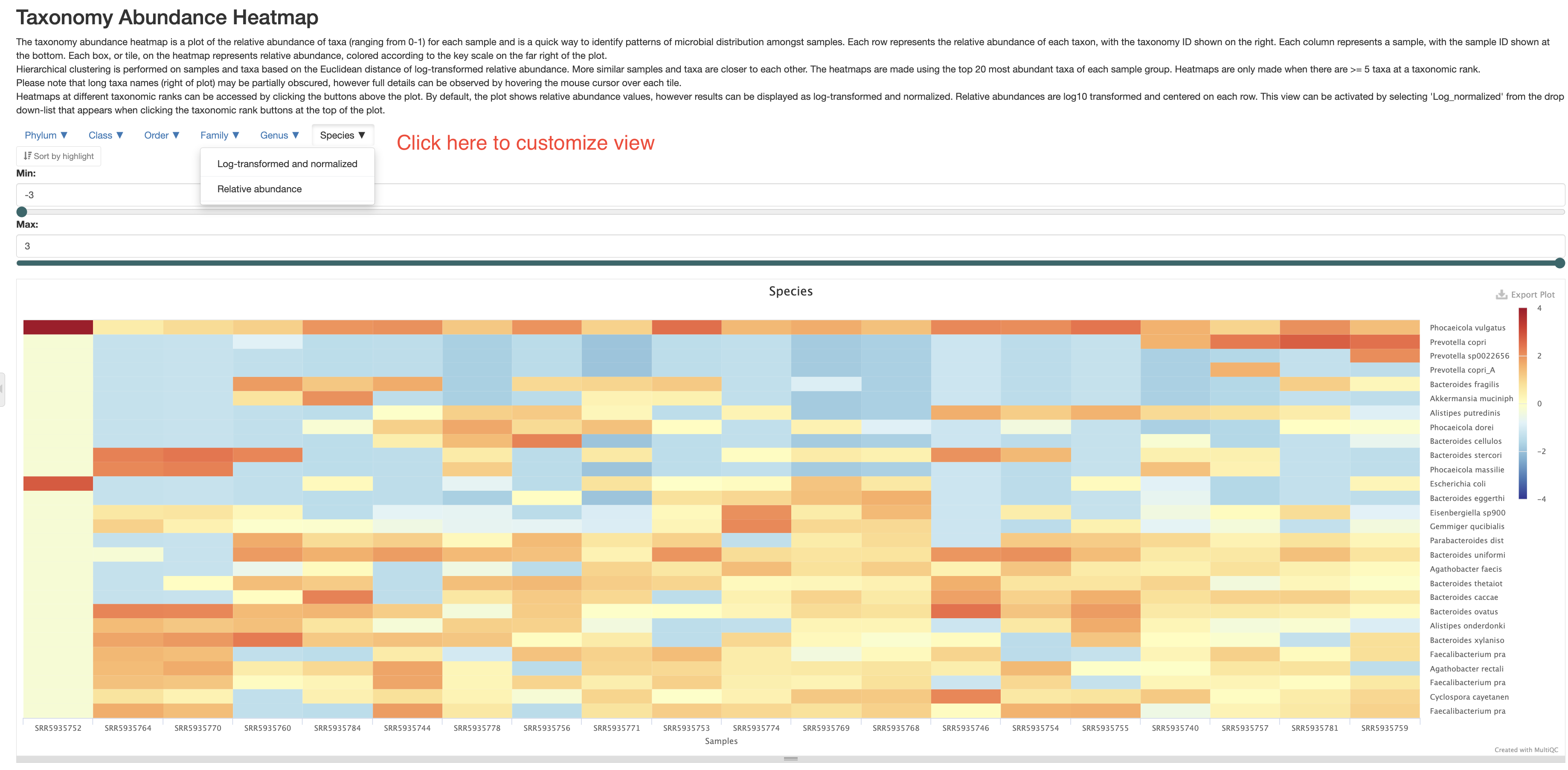
Taxonomy abundance heatmap
This section presents the taxonomy abundance data in a heatmap format. This allows you to see the differences between the samples better. Please note, the heatmap only includes up to the top 20 most abundant taxa at a given taxonomy level. There are two different views of this heatmap. One is of relative abundance, in other words, same data as presented in the “Qiime composition barplot” section, this is useful when you want to know the actual percentages of a given taxa. The other is log-transformed and centered data, which contrasts the changes between samples and taxa better. You can also customize the view at different taxonomy levels.
Diversity analysis
By default, these analyses are carried out at species level, unless specified otherwise when the pipeline was run.
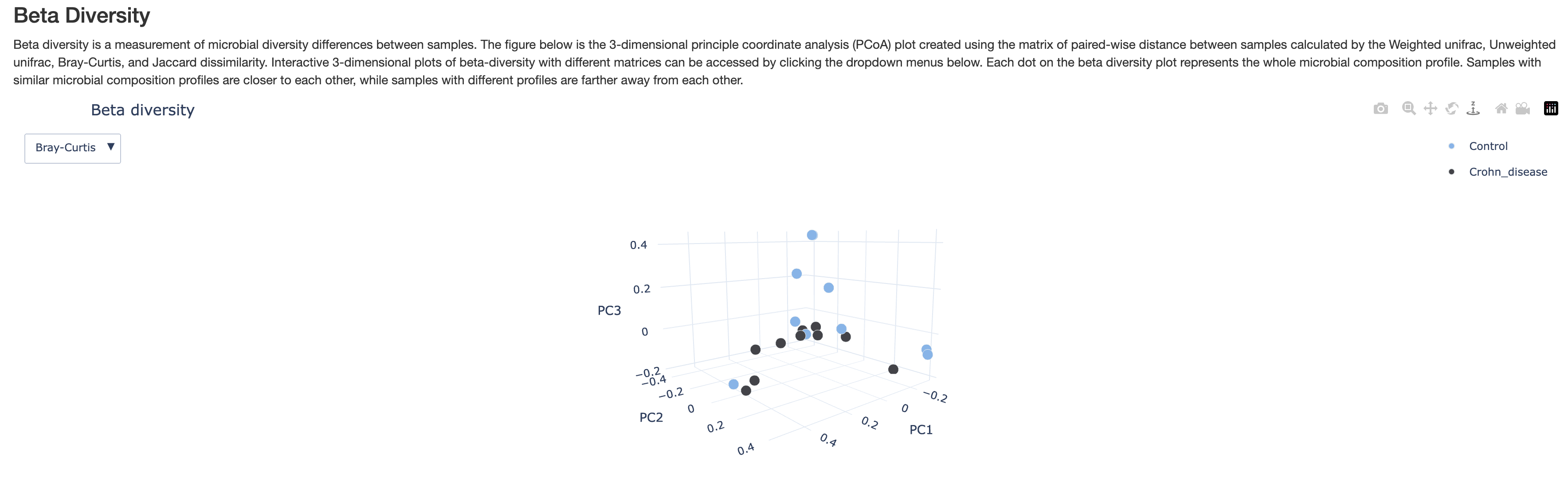
Beta Diversity
This 3-D plot displays principle coordinate analysis results calculated using the beta diversity distances between samples. There are two different beta diversity metrics. You can spin the plot to view at different angles. This plot is intended to show you how similar or disimilar are samples of different groups. You may expect samples of the same group to be more similar to each other, but that may not always be true.
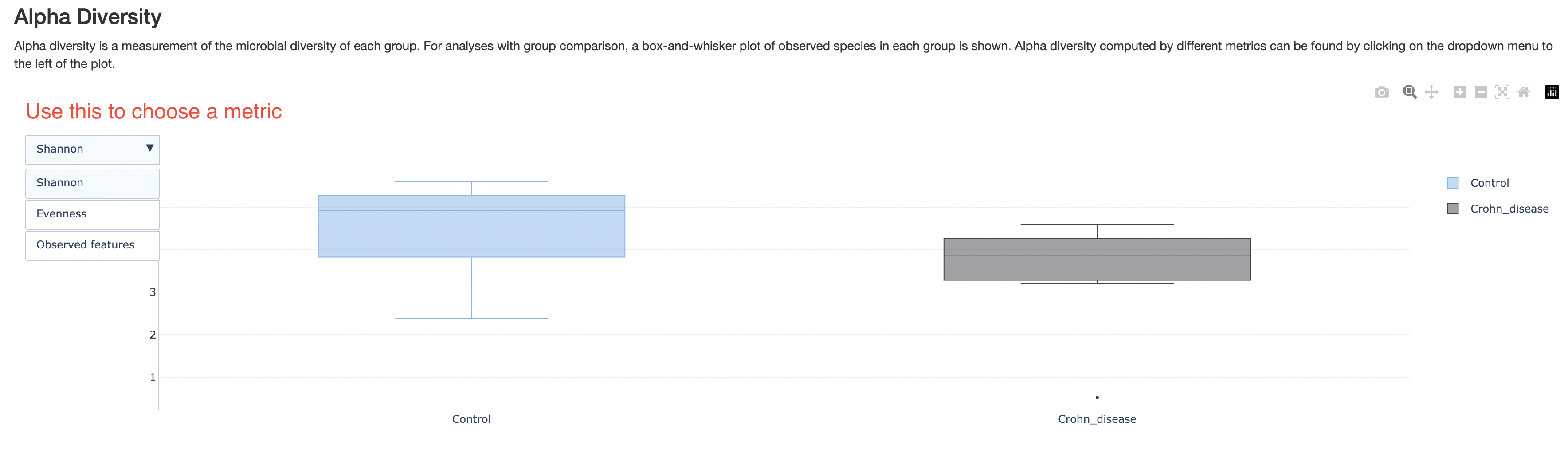
Alpha diversity
This section shows a box plot of the alpha diversities of samples in each group, if group labels are provided. There are three different metrics for alpha diversity. Those can be chosen with the dropdown box on the top left corner. This plot intends to show whether one group of samples is more diverse than others.
Alpha rarefaction
This section shows changes in diversity in each group if there were fewer reads. This is only about species diversity, but shotgun sequencing is more than that, it often tries to detect genomic contents of the species as well. This may be removed in the future version.
Differential abundance analyses
The objective of differential abundance analysis is to identify taxa which differ statistically between samples and sample groups (if this information is provided). The method employed is ANCOM-BC (Analysis of Compositions of Microbiomes with Bias Correction). ANCOM-BC estimates unknown sampling fractions (ratio of the observed taxa abundance in a sample to its actual abundance in the ecosystem) and uses this estimate to correct for sampling bias through a log-linear regression model. Results can be visualized in the ANCOM-BC barplot.
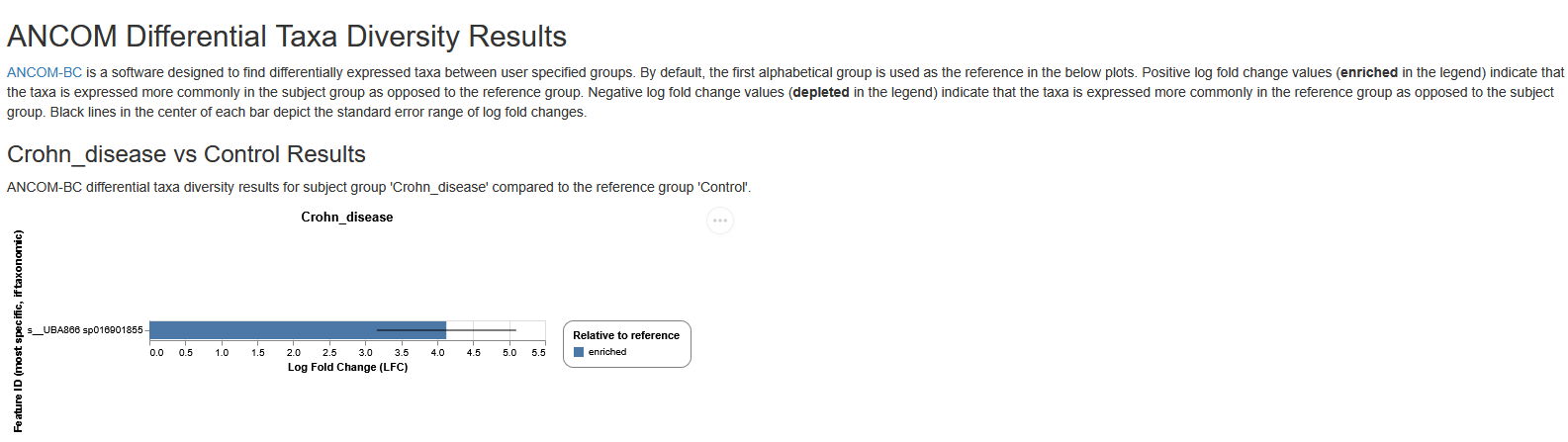
ANCOM-BC barplot
In the ANCOM-BC barplot, the Y-axis represents each taxa and the X-axis represents the log fold change (LFC). Each bar represents the LFC of the absolute abundance of a taxon between treatment groups. Only the significantly differentially abundant taxa are shown (multiple testing adjusted P value (q value) < 0.05). In the subheading above each plot in the format of “Group A vs Group B”, “Group B” is the reference group. A positive LFC (LFC > 0) between Group A vs Group B indicates that relative abundance is significantly higher in Group A relative to Group B, whilst a negative LFC (LFC < 0) between Group A vs Group B indicates that relative abundance is significantly lower in group A. For example, in a ‘Treatment-A vs Control’ comparison, a negative LFC for taxa Escherichia coli indicates that the abundance of this taxa is significantly lower in the Treatment-A group relative to the control group (in other words, the abundance of this taxa is higher in the Control group).
Antimicrobial Resistance Analysis
This pipeline uses AMR++ v3.0 to produce antimicrobial statistics from read alignments to the resistome database MEGARes. MEGARes contains entries for SNP, insertion, and deletion mutations documented to confer antimicrobial resistance, as well as gene sequences that confer antimicrobial resistance by presence. AMR++ compiles counts for MEGARes entries into hierarchical levels; on the first level, gene sequences and mutations that confer anti-microbial resistance, the second, gene groups under which MEGARes entries may be classified, the third, mechanisms targeted by antimicrobial resistance groups, and fourth, to classes under which antimicrobial resistance mechanisms fall. As an example, accession MEG_3779 documents a specific mutation within the MECA gene group, which, along with other groups, encodes the mechanism Penicillin-binding protein, and finally the class betalactams. This pipeline currently produces output for class, mechanism, and gene level data.
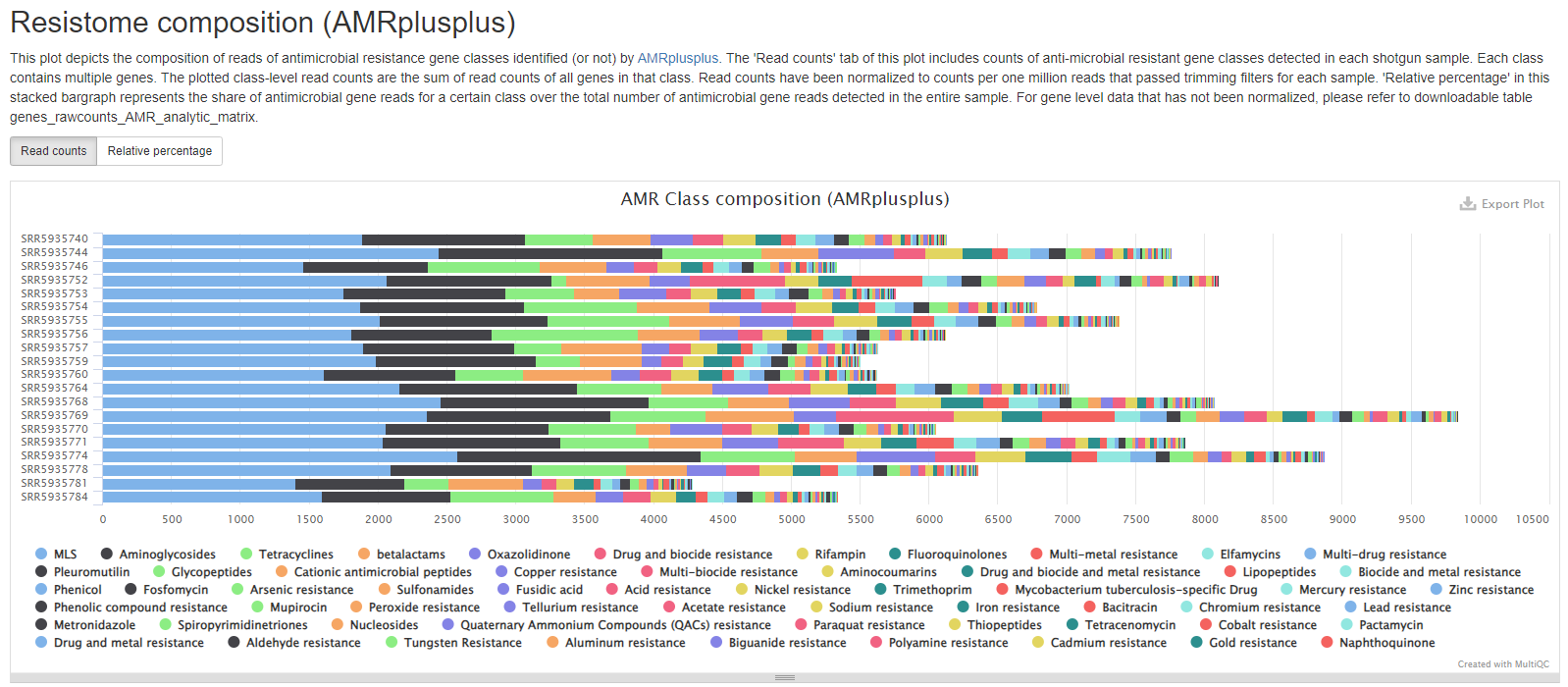
Resistance Class Summary
This plot depicts the read composition of antimicrobial resistance classes. This plot contains two tabs. The ‘Read counts’ tab will give you sample level read counts for all classes. To facilitate comparison between samples that may have different numbers of total reads, read counts have been normalized to counts per one million reads that passed trimming filters for each sample. The ‘Relative percentage’ tab contains the read counts of individual classes divided by the total number of antimicrobial gene reads within each sample.
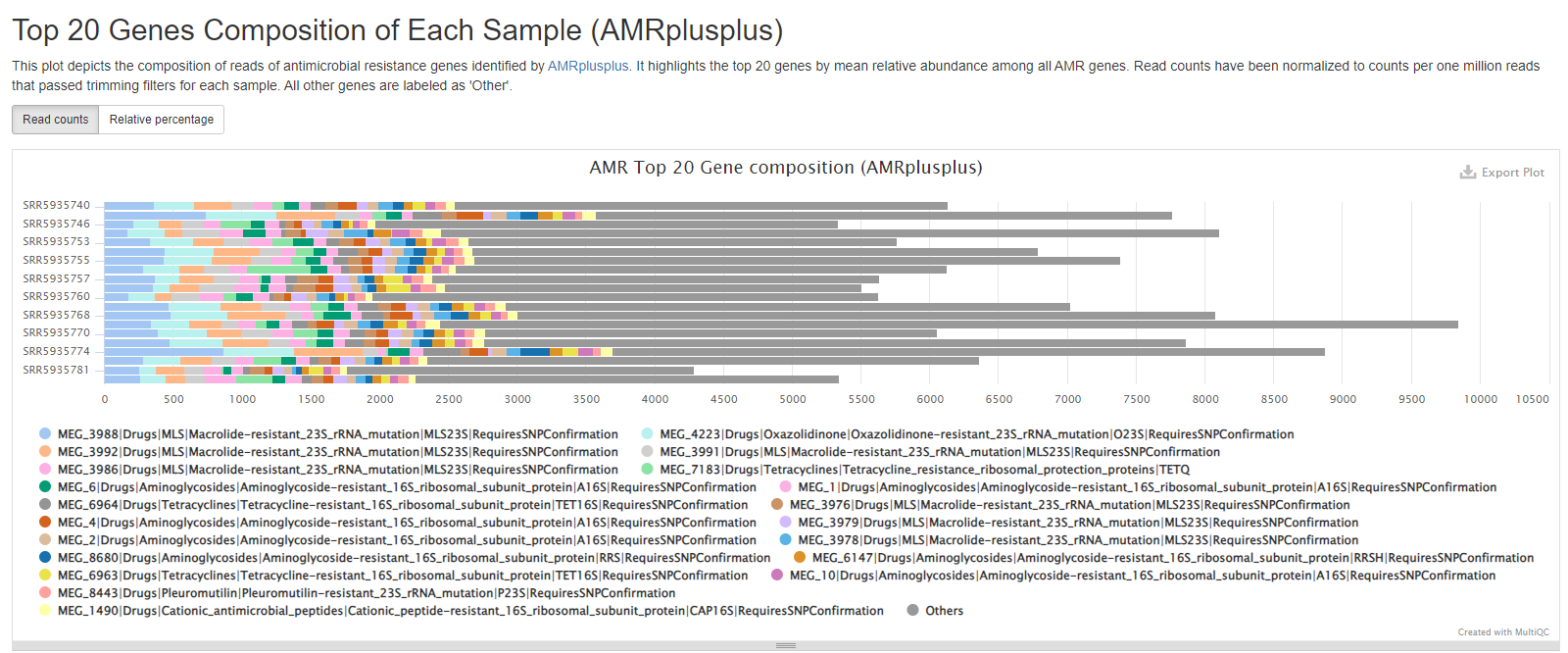
Top 20 Antimicrobial Resistance Genes Composition
This barchart displays the top 20 MEGARes entries ordered by normalized read count for each sample. All other matches to the MEGARes database outside of the top 20 have been grouped into the section “Other”. MEGARes entries contain both SNP mutations documented to confer antimicrobial resistance and gene sequences that confer antimicrobial resistance by presence. The ‘Read counts’ tab contains absolute counts that have been normalized to counts per one million reads that passed trimming. The ‘Relative percentage’ tab includes the number of reads per MEGARes entry divided by the total number of reads aligned successfully to the MEGARes database.
Sample processing
FastQC/Falco
There are two FastQC/Falco sections in the report, a pre-trimming and a post-trimming one. They contain charts and stats about the quality of the reads before and after read trimming step. If the library and sequencing qualities are good, these two sections are usually highly similar. In short, they contain the following subsections:
- Sequence Counts: number of unique and duplicate reads. It is normal to have some duplicate reads, as long as they don’t account for more than half of the reads. Even then, it could be explained by the nature of the sample, for example, low complexity samples, low DNA input, etc.
- Sequence Quality Histograms: quality scores of the reads at different positions. You want most of the reads to have above Q30, but if there are low quality at the ends in the pre-trimming plot, don’t worry, they will get trimmed.
- Per Sequence Quality Scores: average quality scores of the reads. You want most reads to be above Q30.
- Per Base Sequence Content: a composition of the nucleotides at each position. Ideally, they should be pretty even throughout, however, it is normal to have some uneveness at the 5’ or 3’ region because of bias or artifacts in the library kits. As long as all samples in your study have similar behavior, this is usually acceptable.
- Per Sequence GC content: GC content distribution of reads. Because a metagenome samples consist of different organisms of different GC contents, and when some of them account for a large percentage of the community, they may cause peaks in the GC content plot. Therefore, it is normal to have warnings in this plot for metagenome samples.
- Per Base N content: N% in the reads. This should be very low, otherwise your data have poor quality.
- Sequence Length Distribution: distribution of read length. Pre-trimming, the read lengths should be pretty uniform. Post-trimming, if your reads have a lot of adapters, they could vary in lengths.
- Sequence Duplication Levels: %reads with duplication levels. Most duplicated reads are expected to have low duplication levels, if you see samples with significant percentages of reads with high duplication levels, it could indicate a problem in library prep. However, sometimes it could be explained by a really dominant species in the sample.
- Overrepresented sequences: You shouldn’t expect these except for adapter sequences. If you see warnings, check the next subsection.
- Adapter Content: a plot of adapter content at different positions, or a statement when adapter contents are low. High adapter content indicates short inserts in the sequencing library, which you should work to avoid in the library prep protocol. However, when this happens, unless the adpater contents are very very high, or your reads are short to begin with, the data should still be usable. There should be no adapter left in the post-trimming section though.
- Status Checks: a summary of the above plots, red and yellow indicates different levels of warning. However, as we discussed above, some of the warnings are because of the nature of the metagenome samples. Warnings do not necessarily indicate problems. Please read other sections of the report to see if you can find explanations for these warnings.
You can find more detailed explanations of FastQC reports here.
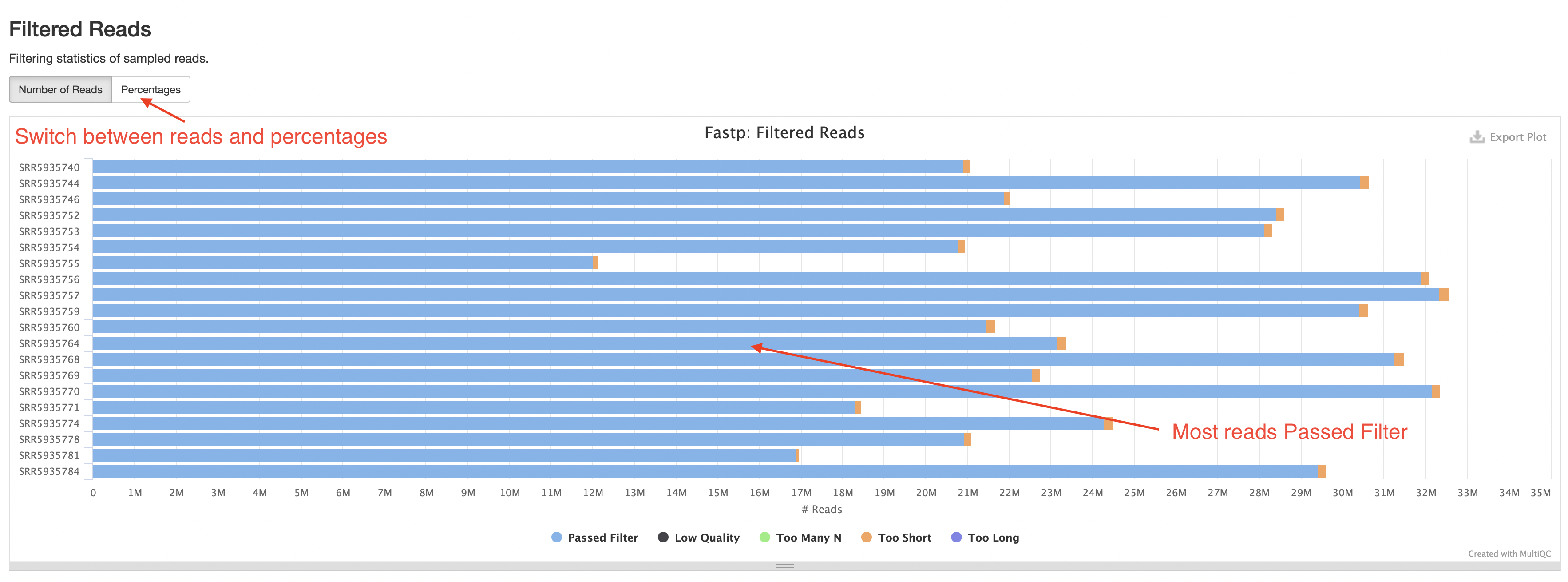
fastp
The fastp section of the report contains stats on the number of reads left over after filtering and an insert size plot. The most important plot here is the “Filtered Reads” barplot. You should expect most reads to be “Passed Filter”.
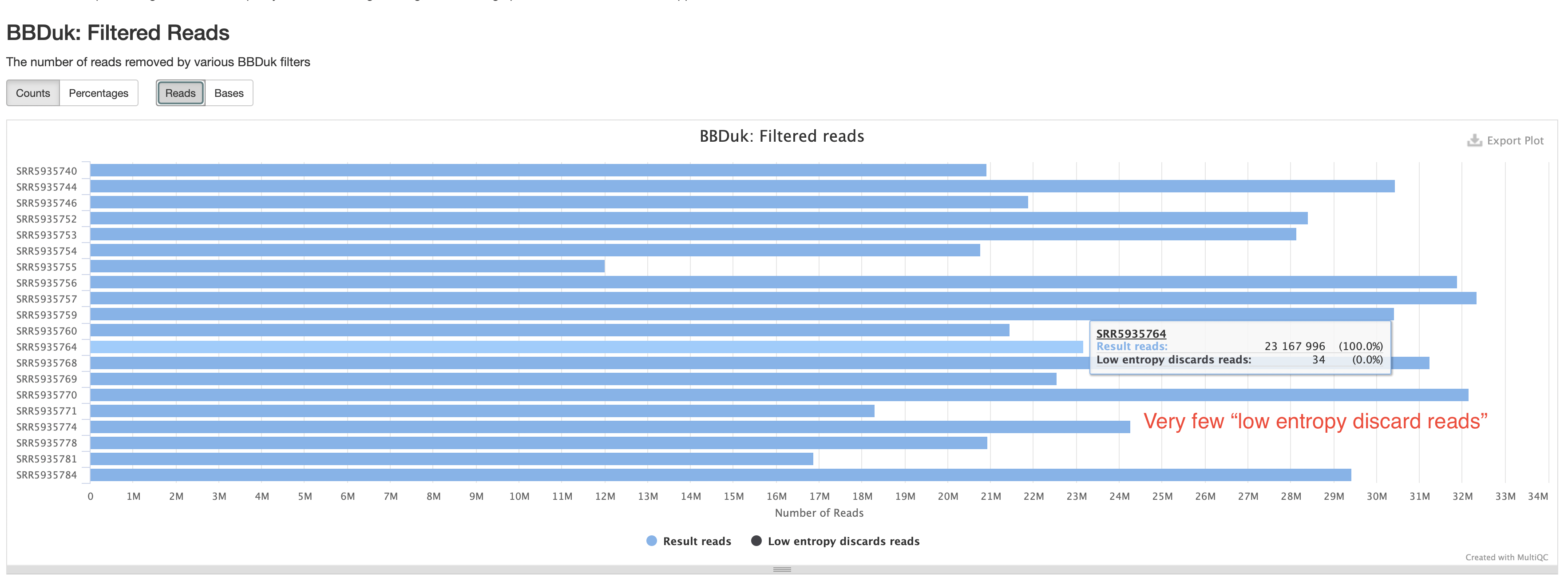
BBDuk
The BBDuk section summarizes how many reads are filtered out because of low complexity or “low entropy”. These reads are highly likely to be derived from technical artifacts. You should expect the percentages of such reads to be very low.
Pipeline information
Software versions
This section lists the versions of software used in this bioinformatic pipeline. This should help you in writing the methods section of your publication or if you wish to carry out some of the analysis on your own.
Workflow summary
This section lists any parameter that were different from the default values. For default values, please refer to the pipeline code.